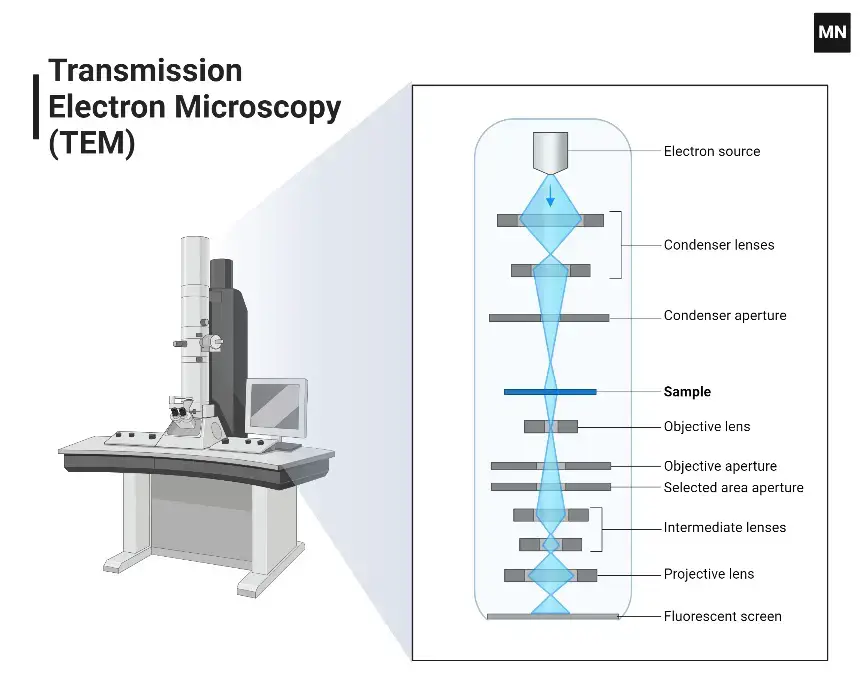
“I just decided to publish my story here and see what happens…I woke up one morning, and I was getting so many people clapping. One person had commented. And then I started noticing people highlighting deep into the article. It was a 2,000-word article!”
Behavioral scientist
Maria Keckler, Ph.D. came to Medium because she had a story she wanted to share with the world and didn’t want to wait for the slower traditional publishing route.
Since then, she’s reached “decision makers, directors, VPs, people leading, UX designers,” on and off Medium.
I enjoyed her write-up of her experience on Medium, so I reached out to ask her to speak with me. Here’s what we discussed:
Table of Contents
· Dr. Maria Keckler, please introduce yourself.
· Tell me about your background, your research, and writing on Medium
· What brought you to write on Medium?
· How does writing on Medium compare with elsewhere online?
· What has been your proudest Medium accomplishment since you started writing here?
· What’s your writing process?
· How do you simplify technical concepts in your writing?
· What is your favorite Medium feature?
· Do you have any tips for new writers on Medium who want to share their story?
This is a lightly edited transcript of our interview.
Want to watch the whole interview? Check it out below:
https://cdn.embedly.com/widgets/media.html?src=https%3A%2F%2Fwww.youtube.com%2Fembed%2F4rOWArniUDw%3Ffeature%3Doembed&display_name=YouTube&url=https%3A%2F%2Fwww.youtube.com%2Fwatch%3Fv%3D4rOWArniUDw&image=https%3A%2F%2Fi.ytimg.com%2Fvi%2F4rOWArniUDw%2Fhqdefault.jpg&type=text%2Fhtml&schema=youtube
Zulie Rane: Maria Keckler, Ph.D., please introduce yourself.
Dr. Maria Keckler: Thank you so much, Zulie. Such a delight being here with you. I am Maria Keckler. I am a research scientist. I am also a writer and storyteller. I love telling great stories to make academic research more accessible and share some of the insights that all of us can apply to our lives.
Tell me about your background, your research, and writing on Medium
MK: For me, writing comes naturally. Before becoming a research scientist and academic, I was in communications for twenty years. Even to this day, I work with companies or executives helping them extract the story behind their message.
I’m always waving the banner: Please tell a story. What is the story? People may think I sound like a broken record. For me, the story is just crucial.
My research is about the neurobiology of storytelling. We know from research that when we communicate with stories, there is activity in our brain that helps us connect, be more empathetic towards each other, and trust each other.
In a previous life, I was a technical writer. One of the things that we can learn from great storytellers and technical writers is that it comes down to the audience first.
If we don’t think about the person on the other side reading it, then we’re just having a monologue.
Number one, that writing is not as fun. Number two, that won’t reach an audience, and it doesn’t have as high an impact on their lives.
What brought you to write on Medium?
MK: Rebranding soft skills to structural skills. For many years, even decades, I have been saying we need to stop calling them soft skills. These are the skills of communication, leadership. These are critical skills that we need every single day.
As an immigrant, it was my soft skills and working in a very focused way on the soft skills that helped me get ahead and advance in my career.
As I started thinking about how we can change how we see them, I thought we should call them structural skills.
I decided to submit an article to a well-known publication. I had a lot of dialogue when I posted about it on LinkedIn, so I thought, great, let’s publish an article about it.
And I didn’t hear anything.
Crickets.
For those of us who chase the traditional publication model in academia, that’s normal. We just look for another publisher.
But today, with change being so fast, we cannot afford to wait around for somebody to tell us we have permission to publish something.
I had been curious about Medium because I read here. I have always been impressed with the writing. The visual design feels very elegant to me.
I am impressed that here, people actually read long form. When you spend a lot of time on LinkedIn or social media, you begin to believe that people no longer read. If you don’t put it into three bullets or 10 points or two tips, people don’t want to read anymore.
On Medium, there was an audience for long-form writing.
I just decided to publish that story here and see what happens. Then I thought, I’ll write an article for each one of those skills I mentioned in my article.
When one of my stories was Boosted by Medium, I woke up one morning, and I was getting so many people clapping. One person had commented. And then I started noticing people highlighting deep into the article. It was a 2,000-word article!
Then I saw someone saved it on this List called, “You need to read.” Another one saved it to a List called “Career.”
As a researcher, I thought, oh my gosh. This is incredible data, how people are absorbing this information, where they’re storing it, and how they’re engaging with it. I was blown away. It’s been surreal to see how many people have engaged with it.
How does writing on Medium compare with elsewhere online?
MK: I had a blog many years ago. Two things were so frustrating. Number one, finding an audience because it takes a long time. Number two, if something broke in the blog, I had to find somebody to fix it. It was a lot of maintenance, worrying about whether it was gonna work, how am I going to bring people here. And I began to lose my true love, which is writing.
On Medium, connecting with people has been so rewarding. Readers highlighting, engaging, all of that.

And then I started noticing that people that I wanted to reach — decision makers, directors, VPs, people leading, UX design — people that I want to work with, people I want to meet? They started sharing the articles on their own LinkedIn profiles. They would tag me and start a conversation. “I’ve been talking about this, and Maria frames it this way.”
I thought, oh my goodness. These people were from all over the world, which is another thing that was just remarkable to me in Medium, how it attracts people from all over the world.
I started thanking readers for sharing it — and then asking to learn more about what resonated, how it applied to their industry.
Just last week, I was on these virtual coffees with somebody from South Africa, from Australia, from the UK, from Glasgow, from Kenya, and across the US. I’ve already met with 15 people.
ZR: Wow. That’s almost every continent.
MK: To me, being able to write something that gives me real feedback and to see which ones are resonating versus others — it’s a little experiment.
Then to be able to see what resonates and to have a way to connect with those individuals is amazing. It’s been very, very fun.
I started publishing without a paywall. Then I thought, you know what? I’m gonna see what it would look like to turn on the paywall. I turned on the paywall, and just like that, I already paid for my Friend of Medium membership.
I’ve also enjoyed meeting other academics who are making an effort to make their writing accessible. That has been really wonderful, which motivates me to bring more of my colleagues to the platform for sure.
What has been your proudest Medium accomplishment since you started writing here?
MK: You know, it has made me want to write. In a month and a half, I’ve published nine or 10 articles that I’ve been tinkering with for a long time, that I’ve had on my notes or Google documents.
I didn’t just start from scratch. I’m a writer, and so I’m always writing. I have so much content, but it’s always been, well, one day, I will make this into a book. Someday, I will do this.
The thought of constantly having to pitch to publications, it just is so laborious when I have so much work with my research, with my business, and then I wanna write.
For me, the discipline has been my proudest moment because I can go grab a piece that I’ve been working on for a long time and say, you know what? I’m gonna finish it.
I have 860 people who decided they want to follow my work. I started a publication to organize these articles, and over 500 people have decided to subscribe to that in a little bit over a month and a half.
I feel like these people want to know what’s the next piece. It inspires me to show up with that value add for them because they took the time to say, “Yes. I care about what you’re writing. I care about craft as well.”
I also find the comments very inspiring — people are truly engaging in conversations. I respond to every comment.
I have also found that I want to read what other people are writing. I want to comment; I want to be generous in that way because I don’t want to just show up to take, you know, read my stuff. I also want to engage with the work, and I found some phenomenal pieces.
I love these writers:
One of the pieces that surprised me was by
James Horton, Ph.D. He’s also a social scientist, and his piece was featured by Medium in Staff Picks: Things You Learn from Skimming 1,350 Academic Journal Articles.
I was so blown away by the writing and visual storytelling, how he used AI to create some whimsical dividers through his story. I just found it so phenomenal.
I wanted to read more of what he’s writing.
It’s just a perfect example of someone who is writing about something that could potentially be a drag to read, but you can relate to it. It is a wonderful perspective to bring to readers.
What’s your writing process?
MK: A lot of the writing process first happens in my head. I am thinking about it constantly. Right now, I’m thinking of my next piece about pivotal optimism as another core skill that we need today.
I ask myself, what is the story that I want to tell that is very personal? What books have I read that talk about it? Is there some data point? How do I pass the ball to the reader so they can see this relates to them?
For me, that process is mental.
I take notes in my Apple Notes. I read. I go to the library, I’ll find a book, grab a quote.
Once that finishes organically, I use a free write device, which is like a keyboard with no Internet. I just write and it goes into the cloud, so that I am not distracted by Internet, by notifications, by AI.
I do a brain dump of everything. It’s very freeing because it’s just the first draft.
I came to the United States as a new immigrant when I was sixteen. I was born in Mexico City. I couldn’t speak English, but I was a really good typist on the old traditional typewriter. In those days, they had a typing class. There was a competition for typing accuracy and speed. I didn’t have to understand what I was typing. I was just sight typing, and I won an award for typing. It was just so cool. That concept really spoke to me of really having the freedom to just type without worrying about the end product.
Once I have a pretty good copy, then I go to AI and ask it to give me a proofread, and tell me what I am missing.
I try to use AI almost like an editor, like someone would use a traditional editor. For me, AI is great for feedback. It’s never good for truly finishing a piece. It almost feels like it wants to take over my voice.
Once I decide that I want to finish a piece, it’s all-consuming for me, even if I have other pieces going on. I go in and I get very obsessive.
How do you simplify technical concepts in your writing?
MK: I try to explain it as I would to an eight-year-old. It forces you to go from an abstract, complicated, technical concept in a way that you would explain it to your own child, a grandchild, a niece or a nephew.
If you’re able to do that, you’re going to discover that part of you that can tell that story. If that becomes too hard for you, that’s a great use for AI. Ask, how would you explain this to an eight-year-old? Just to get your brain in that space. From there, you can begin crafting the story based on that simple concept.
Social scientists or those in the hard sciences, we have such incredible work that should be read by more people. Not just the few who are getting academic articles and can understand them. My dream is that more of them come to bring that value to Medium, and share what has been maybe kept in a vault for a long time.
What is your favorite Medium feature?
MK: I really love the Friend link because I can send my writing to people. I keep a list of my Friend links and send them out to people. It just feels very generous to do that.
All my stories were free originally. I didn’t know they were gonna go viral. I just wanted to get it out there.
But now, I paywall them and share Friend links because I want to be generous with Medium and its readers. I know there is that generosity to the people that are not Medium members, that I want to invite into my writing. I also want to be generous to the process of the platform and, and honor how they have created this way to reward writers.
It just gives me a great way to be generous in multiple ways.
Do you have any tips for new writers on Medium who want to share their story?
MK: Yes. I would say start. You just have to start.
For me, having this thirty-day experiment, I had low expectations and just did it, and I just wanted to learn something from the process.
I would say just do that. Give yourself thirty days and publish a few articles. Try to see it as a true experiment where you are not discouraged if you don’t have a viral moment.
If your first piece, second piece is not going viral, if you don’t have a thousand claps, if you don’t have all of that, it’s still very valuable.
It teaches you how to write in such a way that connects with the audience. If people are not reacting to it, they’re not connecting? That’s great data. Maybe it’s your heading, maybe it’s your title, maybe it’s the storytelling techniques, maybe it’s a topic. Whatever it is, it’s an incredible way to learn how to be a better writer and storyteller and how to connect with your audience.
Use it to hone your writing, to connect with people you have been wanting to reach, and then improve from there.
Learn about your voice. I’ve read a lot on Medium about how to get more views, how to write more articles, how to get more clicks — things that are very practical. But at the end of the day, I do not want to gain followers or get clicks by writing as someone else.
I could build a huge audience by not being me. But the benefit of writing is just being who you are. Oftentimes, we have to go back to the moments in our story that give us those clues about who we are.
In summary: Just show up as the most authentic you. Learn the craft to do it well. Then you’ll begin to attract the people who are looking for you.