The Confocal Microscope, like most advanced imaging systems, can be distinguished by its use of laser light to generate high-resolution images at varying depths within samples. These microscopes, which operate on the principle of confocal laser scanning microscopy (CLSM), then utilize a focused laser beam to scan specimens and detect fluorescence emissions at different depths.
It can be noted that the concept was initially developed by Marvin Minsky during the 1950s at Harvard University. The use of this technology, however, remained limited until sufficient light sources and computerised systems became available to manage the extensive data generated. Generally, the work was next adapted by David Egger M. and Mojmir Petran, who have developed a multiple-beam confocal microscope incorporating a Nipkow spinning disk – which they employed to examine unstained brain tissues and ganglion cells.
The use of confocal microscopy has expanded significantly across various fields. This includes biological research, medical investigations, and materials science, where they have become instrumental in studying cellular structures, tissue compositions, and molecular interactions at microscopic levels. Until the development of solid-state lasers and charge-coupled device (CCD) cameras occurred in the 1980s, the widespread adoption of this technology remained limited.
Like most significant technological advances, the modern confocal Microscope integrates various components. These include optical elements that perform the primary configuration function through electronic detectors, sophisticated computer systems, and laser arrangements. When a person can’t achieve high-resolution, three-dimensional imaging through conventional microscopy, they turn to confocal microscopy, which accomplishes this by scanning samples in sequential optical sections.
The first commercial confocal microscope emerged in 1987, equipped with enhanced optics, electronics, and high-efficiency scanning lasers. It is during the 1990s that the development of multi-photon excitation techniques and other advanced imaging methodologies further expanded the capabilities of these systems. They have since become essential tools for investigating molecules, microbial cells, and tissues at unprecedented levels of detail.
Principle of the Confocal Microscope
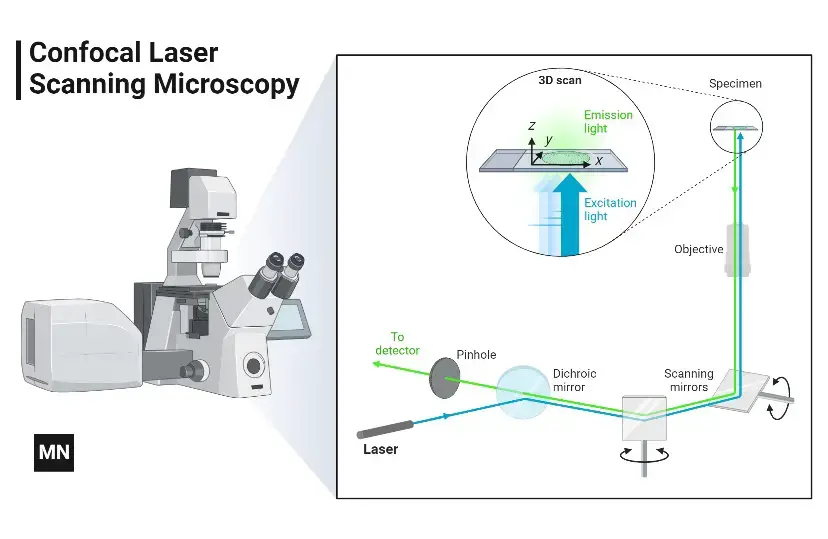
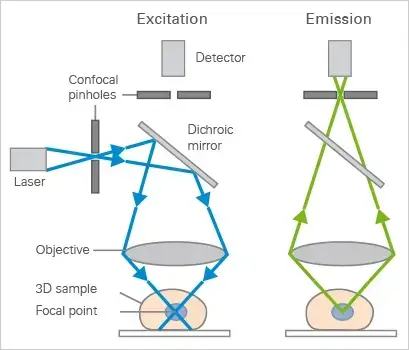
The Confocal microscope Uses point illumination as its principle working mechanism, unlike wide-field or Fluorescent Microscopes which illuminate the whole specimen, receiving complete excitement and emitting light detected by a photodetector. When a beam of light is focused at a particular point of a fluoro-chromatic specimen, it produces an illumination focused by the objective lens to a plane above the objectives- The objective has an aperture on the focal plane located above it, which primarily functions to block any stray light from reaching the specimen.
The illumination point measures about 0.25 to 0.8 um in diameter, determined by the objective numerical aperture and 0.5 to 1.5 um deep, with the brightest intensity. The specimen normally lies between the camera lens and the perfect point of focus, known as the plane of focus.
Using the laser from the microscope, the laser scans over a plane on the specimen (beam scanning0 or by moving the stage (stage scanning). A detector then will measure the illumination producing an image of the optical section. scanning several optical sections, they are collected in a computerized system as data, forming a 3D image which can be measured and quantified. Its outcome is also favoured by the aperture found above the objective which blocks stray light.
Images produced by the confocal microscope have a very good contract and resolution capacity despite the thickness of the specimen. Images are stored in the high-resolution 3D image of the cell complexes including their structures. The main characteristic of the Confocal Microscope is that it only detects what is focused and anything outside the focus point, appears black.
The image of the specimen is formed when the microscope scanner, scans the focused beam across a selected area with the control of two high-speed oscillating mirrors- Their movement is facilitated by galvanometer motors. One mirror moves the beam from left to right on the lateral X-axis while the second mirror translates the beam along the Y-axis. After a scan on the X-axis, the beam moves rapidly back to the starting point to start a new scan, a process known as flyback. No information is collected during the flyback process, therefore the point of focus, which is the area of interest is what is illuminated by the laser scanner.
Parts of the Confocal Microscope
The Confocal Laser Scanning Microscope is made up of a few key components –
- Objective lens- This focuses the laser light on the specimen and collects the emitted fluorescent light.
- Out-of-focus plane – This is the area above and below the focal plane that appears blurred in the image.
- In-focus plane: The plane within the specimen that is in sharp focus.
- Beam splitters – These are used to separate the excitation light from the emitted fluorescence light. They reflect the laser light onto the specimen and allow the emitted light to pass through to the detector.
- Detector – It detects the emitted fluorescent light and converts it into an electrical signal that can be processed to form an image.
- Confocal pinhole (aperture)- A small aperture placed in front of the detector that blocks out-of-focus light, allowing only in-focus light to reach the detector. This results in sharper, more detailed images.
- Laser: Provides the excitation light for the fluorescent dyes in the specimen. Different lasers can be used for different dyes.
- Oscillator Mirrors – These are used to scan the laser beam across the specimen in a raster pattern- One mirror moves the beam in the x-axis, while the other moves it in the y-axis, allowing the entire specimen to be scanned point by point.
Types of Confocal Microscope
- Confocal laser scanning Microscope – This type utilises several mirrors for scanning along X and Y axes on the specimen. Through scanning and descanning processes, the image then passes through a pinhole into the detector.
- Spinning disk- Like most confocal variants, it can be identified as the Nipkow disk. These microscopes employ several movable apertures on a disc to scan for spots of light in a parallel manner over a specified plane. Generally, they have reduced excitation energy requirements compared to laser scanning types, which leads to decreased phototoxicity and photobleaching. It can be particularly useful when imaging live cells.
- Dual spinning Disk (Microlens enhanced confocal Microscope)- Until Yokogawa electric developed this advancement, confocal imaging had certain limitations. The use of a second spinning disk with micro-lenses, positioned before the pinhole-containing spinning disk, revolutionised the process. When light passes through these micro-lenses, they capture a broadband of illumination, focusing it into each pinhole. This reduces blocked light at the spinning disk, making these microscopes significantly more sensitive than traditional spinning disks.
- Programmable array Microscope (PAM) – The use of spatial light modulator (SLM) in these microscopes creates a distinctive imaging approach. They have movable apertures containing arrays of pixels with varying opacity, reflectivity, or optical rotation. It can be noted that microelectrochemical mirrors work in conjunction with a charge-coupled device (CCD) camera to capture images.
Mechanism of Confocal Microscope – How does a confocal microscope work?
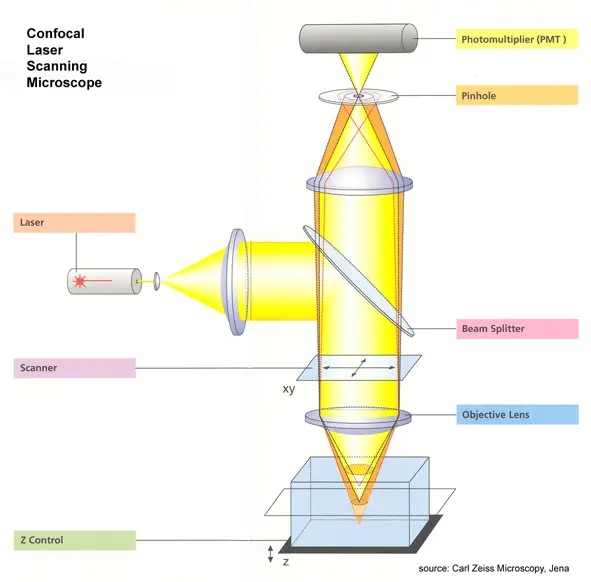
- Confocal, with laser beams rather than lights. It emits laser beams that are concentrated on a fluorescently stained specimen.
- Inserting neutral density filters and a set of scanning mirrors modulate the laser light: the mirrors move very precisely and rapidly.
- One mirror deflects the beam in the X direction, the other in the Y direction. They work in unison to scan the beam in a raster fashion.
- It is then focused by an objective lens onto the sample.
- Once the fluorochrome labeled sample is excited, it will emit fluorescent lights.
- These fluorescent lights will travel into the objective lens the reverse way that the laser travels, that is.
- These scanning mirrors, their main effect on this light is to produce a non-scanning, but stationary, spot of light.
- And then a semitransparent mirror deflects this fluorescent light away from the laser and into the detection system.
- It goes through a pinhole before moving into the detection system. The pinhole only lets through the detectors in the center, a small part of the light.
- Confocal microscopes emit very little light; therefore the light has to be amplified by a photomultiplier tube (PMT).
- Photomultipliers can increase a weak signal by about one million times without adding any noise at all.
- The PMT then sends out an electric signal, and a computer translates this into an image.
Applications of Confocal Microscope
Like most advanced imaging systems, the Confocal microscope can be utilized across diverse scientific domains.
- In Biomedical sciences, it aids researchers when they need to analyse eye corneal infections – this is accomplished through the quantification and qualitative examination of corneal endothelial cells. These specialized cells, which can be murky and difficult to observe with conventional microscopes, become crystal clear under confocal examination.
- Then there’s the microscope’s role in identifying fungal elements within the corneal stroma. This capability proves invaluable during keratomycosis infection, where rapid diagnosis is crucial for therapeutic response. It can be particularly effective when traditional diagnostic methods produce fuzzy or crowded images.
- The use of Confocal microscopy extends into pharmaceutical industries, where they have found it essential for maintaining thin-film pharmaceuticals. This sophisticated tool ensures the quality control and uniformity of drug distribution with a precision ranging from 0.25 to 0.8 um – a level of detail that’s generally impossible to achieve with conventional microscopes.
- These instruments have also revolutionised data retrieval from 3D optical storage systems. It can be noted that this application has proven particularly significant in quantifying the age of historical artifacts, such as the Magdalen’s papyrus. The microscope’s ability to produce detailed, layer-by-layer images at 45°C helps researchers examine ancient documents without causing degradation.
- Through complex imaging techniques and fluorochrome technology, the Confocal microscope continues to advance research across multiple scientific disciplines. Like traditional microscopes, it serves as a fundamental tool in cellular Biology and genetics, whilst simultaneously pushing the boundaries in emerging fields such as Nanoscience and Quantum optics.
The Advantages of Confocal Microscope
- This advanced microscope system can be distinguished by its remarkable capability to produce crystal-clear images. Like most sophisticated imaging devices, they have the unique ability to analyse specimens point-by-point, eliminating scattered light interference that generally clouds traditional microscopic views.
- These microscopes excel in delivering superior resolution, where each focal point is meticulously visualized and captured. It can be particularly useful when examining both live and fixed cell specimens, offering a lot of ease in biological research. The use of confocal systems then allows for collecting serial optical sections, producing detailed cross-sectional views.
- Generally, confocal microscopes ensure uniform illumination across focus points, which helps in producing clear, well-lit specimens. They have the ability to adjust their magnification electronically – without changing objectives – through what’s known as the zoom factor. This feature provides enhanced flexibility during specimen examination.
- The microscope can generate impressive 3D sets of images, allowing researchers to visualize specimens from multiple angles. When a specimen needs to be examined thoroughly, these systems excel at producing detailed volumetric data that traditional microscopes cannot achieve.
- It can be noted that the confocal microscope’s ability to eliminate out-of-focus light results in sharper images with better contract than conventional microscopes. The use of this technology has revolutionised the way researchers examine specimens, offering unprecedented clarity and precision in scientific imaging.
Limitations of Confocal Microscope
- This microscope system has only a limited number of wavelengths, which can definitely be a disadvantage when working with specimens that need multiple fluorescent markers and such markers. Like most high-tech instruments, they have limitations, which compromise their general usefulness in research.
- Well, the expense can become a little troublesome. These things would cost a lot to make and even more to maintain. Labs don’t have money like that to pay it, usually.
- Some are quite advanced, though still hitting the Resolution ceiling. It is a problem because when specimens become over 2 μm or so, images are difficult to obtain because too much fluorescence from specimens obscures detail.
- The optical artifacts are a problem – the self-shadowing effects in thick specimens introduce much difficulty in image interpretation. Then the poor quality objective lenses cause spherical aberrations.
- One should be aware that photobleaching is a significant limiting factor, where the fluorophores lose their ability to fluoresce if the imaging sessions last too long. And therefore compromises the quality of quantitative experiments and longer assays like cell imaging.
- Due to the nature of the microscope and the image acquisition speeds, the. They have limitations, however, that are more evident during live cell imaging. And you cannot really observe real-time cellular processes.
- Those systems have limited versatility because of the nonvarying pinhole sizes, and therefore imaging conditions and performances.
- These finely tuned instruments only operate at a maximum utility level with thinner specimens (i.e., specimens greater than 2 micrometers have poor images).
- Elliott AD. Confocal Microscopy: Principles and Modern Practices. Curr Protoc Cytom. 2020 Mar;92(1):e68. doi: 10.1002/cpcy.68. PMID: 31876974; PMCID: PMC6961134.
- https://bitesizebio.com/19958/what-is-confocal-laser-scanning-microscopy
- https://www.microscopemaster.com/confocal-microscope.html
- https://ibidi.com/content/216-confocal-microscopy
- http://www.physics.emory.edu/faculty/weeks//confocal/
- https://www.olympus-lifescience.com/en/microscope-resource/primer/techniques/confocal/confocalintro/
- https://www.future-science.com/doi/full/10.2144/000112089
- https://www.microscopyu.com/techniques/confocal/introductory-confocal-concepts